Abstract

Introduction
An increased risk of thromboses has been described in patients with autoimmune hemolytic anemia (AIHA), including stroke. Previous studies focused on a broad variety of autoimmune disorders and only adjusted to a limited extent for potential confounders. Further, these studies could not robustly address the relationship of stroke risk with time since AIHA diagnosis. To overcome these shortcomings, we conducted a study of the risk of ischemic stroke amongst patients with primary AIHA in two nationwide cohorts in Denmark and France.
Method
We identified all patients 15+ years of age diagnosed with primary AIHA in the Danish National Patient Register (1996-2016) and the French health insurance database, Système National des Données de Santé (2012 - 2017). At AIHA diagnosis, patients were age- and sex-matched to comparison cohorts from the general populations in Denmark (1:50) and France (1:5), respectively. Each patient's date of diagnosis was allotted to their corresponding comparisons and marked the start of a 5-year follow-up of the cohorts. We linked data on the cohorts to information from national registers concerning (co)morbidity, vital status and outcomes. Based on this data, we identified comorbidities at baseline (including preexisting cardiovascular and cerebrovascular events (CVD) at diagnosis) and ischemic stroke events during follow-up.
We evaluated the absolute risk of ischemic stroke using cumulative incidences, treating death and migration as competing events. Further, the relative risk of ischemic stroke was evaluated using unadjusted cause-specific proportional hazard regression (csHR) and Fine-Gray subdistribution HR (subHR), and subsequently adjusting for CVD event history, atrial fibrillation, diabetes, hypertension, dyslipidemia, history of venous thromboembolism, cancer, and time-varying exposure to antiplatelet, anticoagulant, lipid lowering, and antihypertensive drugs. HRs were estimated both overall for all five years and for the first year after diagnosis for the two countries separately. Hazard ratios for the data from both countries combined were also computed using meta-analysis techniques.
Results
We included 1,171 (Denmark) and 4,823 (France) patients with primary AIHA and 57,773 (Denmark) and 23,752 (France) comparisons from the general population. During follow-up, 35.1% (Denmark) and 38.3% (France) of patients with AIHA died.
Age, gender, and comorbidity distribution as well as mortality was similar in the two countries. Similar differences in the prevalence of previous CVD events were observed between AIHA patients (higher prevalence of CVD) and comparison cohorts in both countries.
We identified 29 (Denmark) and 92 (France) ischemic stroke events amongst patients with AIHA, of whom 12 (Denmark) and 57 (France) occurred during the first year after AIHA diagnosis.
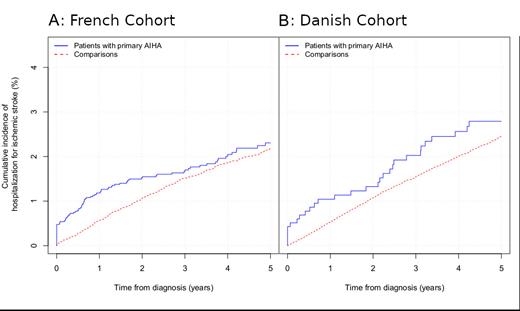
The cumulative stroke incidences are depicted in the Figure (Panel A: French cohort. Panel B: Danish cohort).
Overall, the combined adjusted csHR was 1.3 [1.1; 1.6], and the subHR was 1.0 [0.9; 1.2].
Within the first year from diagnosis, the combined adjusted csHR was 2.3 [1.8; 2.9], and the subHR was 1.7 [1.3; 2.2].
Discussion and conclusion
In a combined Danish-French cohort of 5.994 patients with primary AIHA and 81.525 comparisons, we confirm that the risk of ischemic stroke amongst patients with primary AIHA is increased.
The risk is highest within the first year after diagnosis with a csHR of 2.3 and a subHR of 1.7. This indicates that although the risk of ischemic stroke is high among patients with primary AIHA compared to the general population, the effect on the cumulative incidence of ischemic stroke is diminished by competing events - especially death.
Granular data on treatment was not generally available and our data does not allow us to infer whether the increased risk of ischemic stroke in AIHA derives from the hemolytic process, the treatment or other factors. However, as the risk was highest within the first year and as prior thromboembolic and vascular events were more prevalent amongst patients this could support the hypothesis that free hemoglobin leads to endothelial dysfunction and a procoagulant state that also increases the risk of stroke.
Hansen: Novartis: Research Funding; Alexion: Research Funding. Berentsen: True North Therapeutics: Consultancy; Sanofi: Consultancy, Honoraria; Janssen-Cilag: Honoraria; Bioverativ: Consultancy, Honoraria; Mundipharma: Research Funding; Apellis Pharmaceuticals: Consultancy, Honoraria; Alexion Pharmaceuticals, Inc: Honoraria. Frederiksen: Novartis: Research Funding; Alexion: Research Funding; Gilead: Research Funding; Abbvie: Research Funding; Janssen Pharmaceuticals: Research Funding. Moulis: Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Argenix: Membership on an entity's Board of Directors or advisory committees; Grifols: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sobi: Membership on an entity's Board of Directors or advisory committees.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal